Over the past several decades, dietary fat has often had an undeserved “unhealthy” reputation.
While this mindset is changing in recent times, one particular fat—extra virgin olive oil—has long held a “heart-healthy” reputation.
Furthermore, scientific research widely supports the idea that olive oil is generally beneficial.
However, it is common to hear claims that we shouldn’t use extra virgin olive oil for cooking.
Is there any truth to this?
In this article, we examine this claim in more detail, followed by a look at what the research says about cooking with extra virgin olive oil.

Why do people claim we shouldn’t cook with extra virgin olive oil?
First of all, fatty acids are prone to oxidation from heat, light, and oxygen. For this reason, the process of frying with oil can cause some of the fat to oxidize.
Furthermore, this oxidation process can lead to the production of secondary oxidation products such as aldehydes and other polar compounds. Research shows that these compounds can have adverse effects, and chronic exposure may be detrimental to human health (1, 2).
However, oils which are repeatedly heated are the biggest source of oxidation products (3).
Saturation
Furthermore, the specific fatty acids within a cooking oil play a role in how prone to oxidation it is.
Saturated fats are the most stable fatty acids, hence they are most resistant to oxidation (4).
Since olive oil is predominantly an unsaturated fat, some people claim it is not suitable for cooking.
Smoke point
The smoke point of oil—the temperature at which the oil starts to burn—is another metric that people use to judge the merits of a particular cooking fat.
In this regard, various sources suggest that extra virgin olive oil has a smoke point somewhere between 191°C (376°F) and 215°C (419°F) (5, 6).
This smoke point is much lower than cooking fats such as ghee (250°C/482°F) and refined avocado oil (271°C/520°F) (7, 8).
However, the fatty acids in olive oil are reasonably heat-stable
Is there really a good reason to fear cooking with extra virgin olive oil?
Let’s first look at the oil’s fatty acid breakdown.
According to the USDA Food Composition Database, extra virgin olive oil contains the following amounts of fatty acids per 100 grams (9);
- Saturated Fat: 13.8 g
- Monounsaturated Fat: 73.0 g
- Polyunsaturated Fat: 10.5 g
The Different Saturation Levels of Fat
Firstly, there are three primary groups of fatty acids, and these are as follows;
- Saturated fat
- Monounsaturated fat
- Polyunsaturated fat
All three of these fats contain the elements carbon and hydrogen.
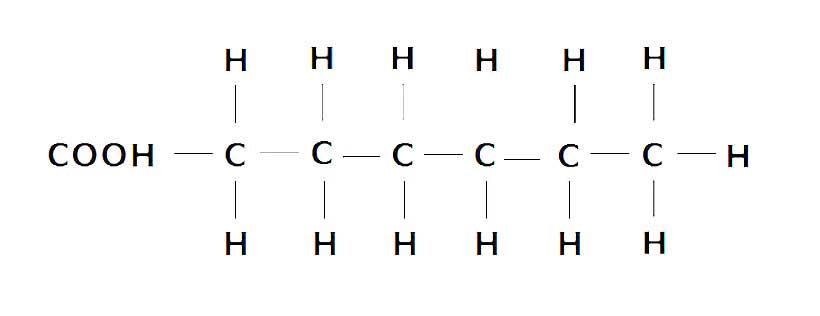
In saturated fat, the carbon atoms are entirely “saturated” (surrounded) by hydrogen atoms, and the fat molecule contains no double bonds.
For this reason, they are very resistant to oxidation (10).
The following diagram shows the molecular structure of saturated fat;
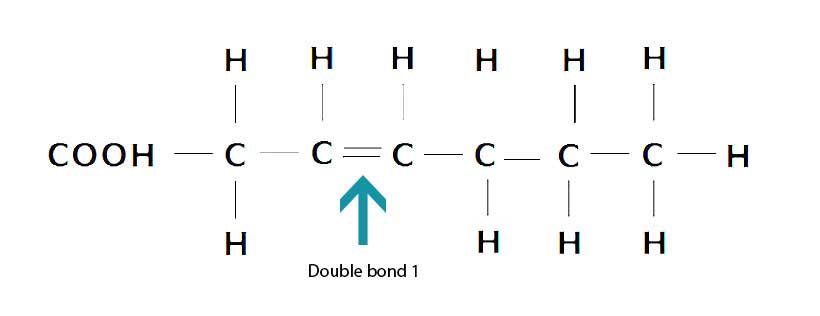
In contrast, monounsaturated fatty acids contain one (mono) carbon-carbon double bond and they are not fully saturated with hydrogen. This double bond can be broken, which makes monounsaturated fat slightly more prone to oxidation than saturated fat (11).
Here is the structure of a monounsaturated fatty acid;
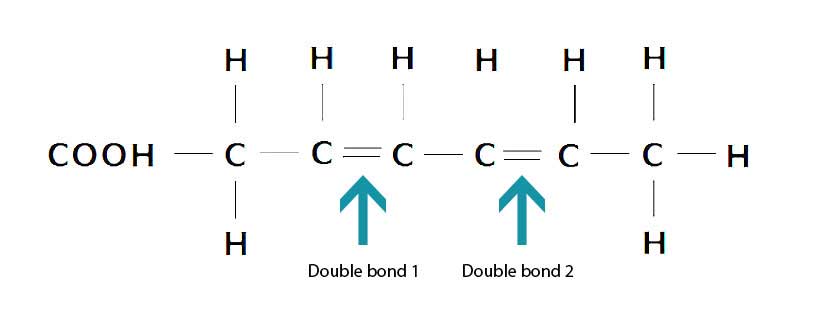
Lastly, polyunsaturated fats contain multiple (poly) double bonds, and they are more prone to oxidation than other fatty acids (12).
The diagram below shows one example of a polyunsaturated fatty acid which, in this case, has two double bonds;
Since olive oil is around 90% saturated and monounsaturated fat, it is fairly resistant to oxidation.
Regarding these fatty acid ratios, it is easy to hear claims that animal fats are heat stable but that olive oil isn’t.
Interestingly though, extra virgin olive oil has a similar fatty acid ratio to lard, and both contain the same amount of polyunsaturated fat – around 10/11 ml per 100 ml (13, 14).
The smoke point of an oil doesn’t represent the oil’s oxidative stability
Many of us judge the relative benefits of cooking oil with a sole focus on the smoke point.
However, research demonstrates that the fatty acids in cooking oil can breakdown before an oil even hits that smoke point.
For example, a recent study compared the chemical and physical changes in ten commercial oils while heating them at a temperature of 180°C (356°F), among various other heat/time tests (15).
These oils were as follows;
- Avocado oil (non-refined)
- Canola oil
- Coconut oil
- Extra virgin olive oil
- Grapeseed oil
- Olive oil
- Peanut oil
- Rice bran oil
- Sunflower oil
- Virgin olive oil
What Did the Study Show?
To directly quote the researchers;
“From this study, it can be concluded that, under different heating conditions, the generation of polar compounds with temperature and time was more pronounced for refined seed oils with higher initial values of smoke point” (16).
For those unaware, the term “seed oil” is another name for common vegetable oils (which are actually from seeds rather than vegetables) such as canola and grapeseed oil.
The table below shows the final amount of polar compounds each oil generated, as well as the oil’s smoke point in the test;
| Type of Cooking Oil | % Final Polar Compounds | Smoke Point (°C) |
|---|---|---|
| Avocado oil (non-refined) | 11.60 | 197°C (387°F) |
| Canola oil | 22.43 | 256°C (493°F) |
| Coconut oil | 9.30 | 191°C (376°F) |
| Extra virgin olive oil | 8.47 | 207°C (405°F) |
| Grapeseed oil | 19.79 | 268°C (514°F) |
| Olive oil | 11.65 | 208°C (406°F) |
| Peanut oil | 10.71 | 226°C (439°F) |
| Rice bran oil | 14.35 | 237°C (459°F) |
| Sunflower oil | 15.57 | 255°C (491°F) |
| Virgin olive oil | 10.71 | 175°C (347°F) |
As shown in the table, the smoke point of each oil had little relevance to the number of oxidation products the oil generated.
In this study, extra virgin olive oil generated a lower amount of polar compounds than any other cooking fat.
Extra virgin olive oil is rich in antioxidants and polyphenols
Another point worthy of consideration is that extra virgin olive oil is a rich source of polyphenols as well as the antioxidant vitamin E.
For example, olive oil contains approximately 2 mg of vitamin E per tablespoon, which is equivalent to around 10% of the recommended daily intake (17).
Vitamin E has antioxidant properties, and research demonstrates that it can increase the resistance of fatty acids to oxidation (18).
Additionally, extra virgin olive oil is one of the richest dietary sources of polyphenols. These specific polyphenols include hydroxytyrosol, tyrosol, oleuropein, and oleocanthal (19).
Initial research suggests that these compounds can also help protect fatty acids from oxidizing (20, 21).
What Does Research Show About Cooking With Olive Oil?
Several studies have tested how olive oil performs when exposed to high-heat cooking temperatures.
Here is a brief summary of some relevant research;
- In one study, researchers used several commercial oils for deep frying and took samples of each oil every three hours. Compared to commercial vegetable oil blends, extra virgin oil had lower levels of oxidation (22).
- Much lower concentrations of aldehydes form when shallow frying fish in extra virgin olive oil compared to sunflower oil (23).
- In one trial, potatoes were deep-fried in either extra virgin olive oil, peanut oil, or canola oil. During the frying process, extra virgin olive oil formed fewer aldehydes and polar compounds than the other two oils (24).
- In a study featuring 20 participants, eating a breakfast fried with extra virgin olive oil led to lower levels of postprandial oxidative stress compared to either canola or sunflower oil (25).
For those looking for a heat-stable oil for long duration, high-heat cooking, it is also worth noting that ‘high oleic’ oils can be a good, and more affordable, option. The seeds used to make these oils are bred to have a high monounsaturated fat content.
For more information, see this guide to high oleic sunflower oil.
Final Thoughts
Extra virgin olive oil is primarily a source of monounsaturated fatty acids.
These fatty acids are reasonably resistant to oxidation, and extra virgin olive oil contains a range of protective antioxidant compounds too.
For these reasons, cooking with olive oil appears to be a reasonably good choice.
However, while olive oil might be a good choice for the kitchen, it is important to buy a good quality oil.
Unfortunately, there have been several scandals with olive oil in recent years. Sadly, there have been cases of inferior oils falsely being passed off as ‘extra virgin’ olive oil.
For more information on trustworthy brands, see this list of olive oils.









Coconut oil was not mentioned. I understand that coconut oil is much healthier than olive oil. Is this correct?
Actually, coconut oil was one of the other cooking oils in one of the test studies (see the table). Either way, they are both fairly heat-stable cooking oils, and each has its own merits.
I’ve read coconut oil has one of the highest smoke points but in the table it ranks lower than Olive oil. Are there different types of coconut oil?
Yes, there are different types of coconut oil – refined coconut oil has a smoke point of around 450°F (232°C).
That said, note that the smoke point doesn’t appear to influence each oil’s oxidative stability, as shown in the referenced study.
For years now I’ve been using virgin olive oil to cook with and much prefer it to other vegetable oils on sale. I’ve never had any issues when using it as a base to fry foods in, ie onions, garlic etc, and roast potatoes basted in virgin olive oil always turn out perfectly.
Glad to hear. Personally, I think it adds a nice flavor to foods too, though some people don’t like the taste.
How does it compare with butter / ghee?
I don’t think they have been compared directly, unfortunately. I would love to see a study like the one referenced that included all the different animal fats. Based solely on personal opinion, I expect ghee would display better oxidative stability, but those who worry about the effect on LDL etc may prefer to use olive oil.
Thanks!
Do you advise against Canola looking at higher oxidation products, or does that just amount to mechanistic speculation not born out by health outcomes?
That’s a good question. While it is true that Canola seems to produce higher amounts of oxidation products, I think it is difficult to view that as being equal to evidence of an adverse impact on human health outcomes.
1) Canola/rapeseed doesn’t seem to be associated with adverse health outcomes based on human trials & observational data.
2) Extremely long/high heat temperatures probably aren’t indicative of how oil is used at home for shallow pan-frying (though perhaps they’re not too dissimilar from industrial processes).
This article was more about focusing on the myth that olive oil shouldn’t be used at heat rather than ranking particular oils as good/bad.